Homoeopathic Pharmacopoeia of India - An Old and Rare Book (Second Volume)
Book Specification
| Item Code: | NAM233 |
| Publisher: | Government of India, Ministry of Health and Family Welfare, Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy, New Delhi |
| Language: | English |
| Edition: | 1984 |
| Pages: | 301 |
| Cover: | Hardcover |
| Other Details | 12.0 inch X 9.0 inch |
| Weight | 680 gm |
Book Description
The Homoeopathic Pharmacopoeia Committee was reconstituted by Government of India, Ministry of Health and Family Welfare vide their letter No. X. 19018/26/79-Homoeo dated 12th November, 1981. The Committee decided to revise the Second Volume to make it up to date before reprinting it. It was also decided to include General Notices, General Instructions, Nineteen appendices of the First Volume of Homoeopathic Pharmacopoeia of India besides appendices of this Volume to make it self sufficient.
The revised (Second Edition) of Homoeopathic Pharmacopoeia of India consists of:- 1. Foreword
2. Preface to Second Edition.
3. Preface to First Edition.
4. Introduction
5. General Notices
6. General Instructions
7. Monographs (100)
8. Appendices
The Second Volume (revised) of the Homoeopathic Pharmacopoeia of India is presented herewith to the Government of India.
The Government of India constituted the Homoeopathic Pharmacopoeia Committee in 1962 for the purpose of preparing the Homoeopathic Pharmacopoeia of India with a view to provide standards for the commonly used Homoeopathic medicines. This Committee did the preliminary work in. preparing a comprehensive list of possible homoeopathic medicines and drafted the general notices and general instructions for the pharmacopoeia. It also prepared 180 monographs comprising the first volume which has since been published.
The first volume of the Homoeopathic Pharmacopoeia of India providing official standards for 180 drugs was finalised in 1970 and has since been published. The second volume, covers another 100 homoeopathic drugs.
The Committee considered it necessary to make certain amendments in the general notices and general instructions contained in the first volume. These general notices are given in this volume. These are applicable to the contents of the first volume also. The format of the monographs has also been slightly changed in the second volume as under:- 1. The heading 'Chemical Symbol' has been deleted and only chemical formula has been given.
2. The heading 'Synonym' has been restricted in case where it relates to a true synonym and in other case it is replaced by common name. In case of chemicals alternate names have however been given without any specific title.
3. Part used has been mentioned after Description.
4. Under the heading 'History and authority' the first prover has been mentioned in the first place and the remaining authorities in alphabetical order.
5. The old Hahnemannian method of preparation has been discarded in favour of new" uniform method with specific drug strength which takes into consideration the moisture content of the drug, thus eliminating variation in standards. This method is applicable to most of the drugs and has been accepted by the Committee.
6. The Title 'Habitat' has been substituted by term 'Distribution', to convey appropriate meaning.
The material compiled in this volume as in the previous one has been freely drawn upon from the British Homoeopathic Pharmacopoeia, American Homoeopathic Pharmacopoeia, the Homoeopathic Pharmacopoeia of United States, the German Homoeopathic Pharmacopoeia and the Indian Pharmacopoeia and other relevant literature.
The Homoeopathic Pharmacopoeia Committee also places on record the appreciation of the work done by the members of the various sub-Committees and the staff in bringing out this Volume.
The Committee is grateful in particular to the Drugs Controller (India), the Director, Central Drugs Laboratory, Calcutta, the Director, Central Indian Pharmacopoeial Laboratory, Ghaziabad and the Director Homoeopathic Pharmacopoeia Laboratory, Ghaziabad who have from time to time offered their valuable suggestions and co-operation.
| Foreword | (i) | |
| Preface to the Second edition | (v) | |
| Preface to the First edition | (vii) | |
| Introduction | (xi) | |
| General Notices | 1 | |
| General Instructions | 5 | |
| List of Monographs with abbreviations | 17 | |
| Monographs | 20 | |
| Appendices | 131 | |
| I. | Material and solutions employed in tests | 133 |
| II. | Solutions employed in Volumetric determinations | 157 |
| III. | Indicators employed in Volumetric determinations and in pH determinations | 159 |
| IV. | Determination of Melting range and boiling range | 164 |
| V. | Determination of Refractive index. | 168 |
| VI. | Determination of weight per ml and specific gravity | 169 |
| VII. | Qualitative reactions of some common substances and radicals | 170 |
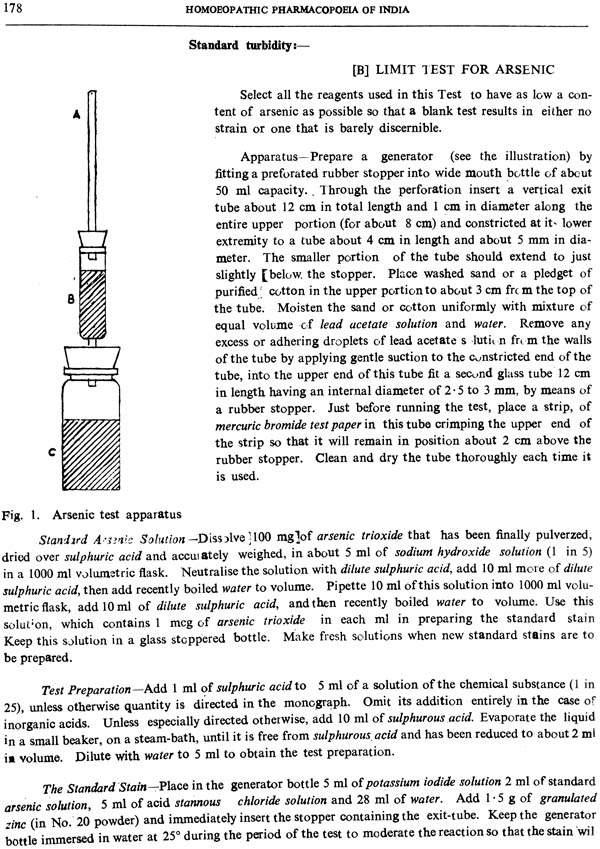
| VIII. | Limit tests | 177 |
| IX. | Determination of ash, salphated ash, water soluble ash and residue on ignition | 182 |
| X. | Moisture content determination | 183 |
| XI. | Determination of alcohol soluble extractive, water soluble extraction and total solids | 184 |
| XII. | Powders and sieves | 185 |
| XIII. | Standards of vehicles used for external applications | 187 |
| XIV. | Determination of saponification, iodine and acid values | 194 |
| XV. | Test for absence of oils | 196 |
| XVI. | Names, symbols and atomic weight of elements | 197 |
| XXA. | Determination of esters | 199 |
| XXB. | Determination of ester value | 200 |
| XXI. | Determination of palisade ratio, stomatal index etc. | 201 |
| XXII. | Specific gravity of solids | 203 |
| Index | 205-208 |