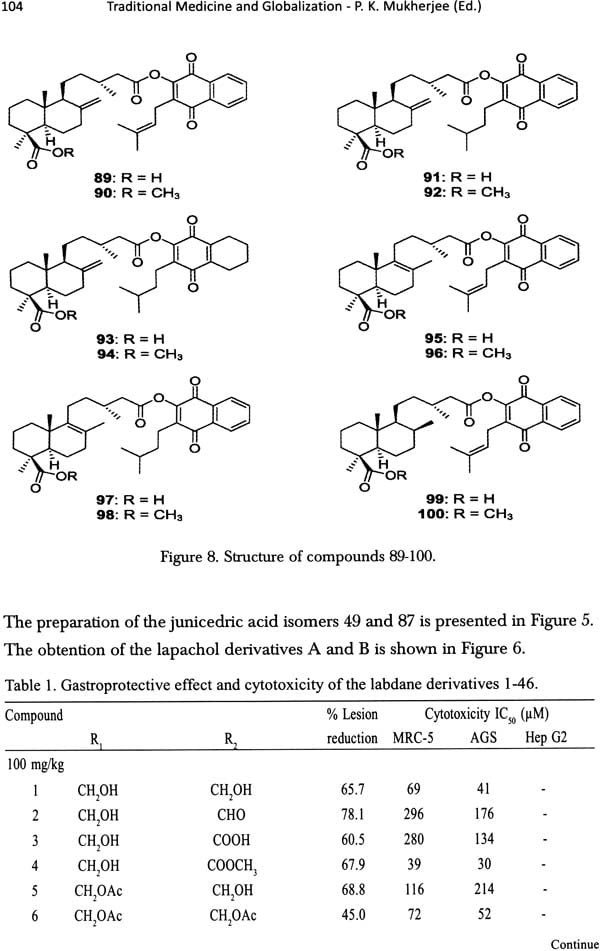
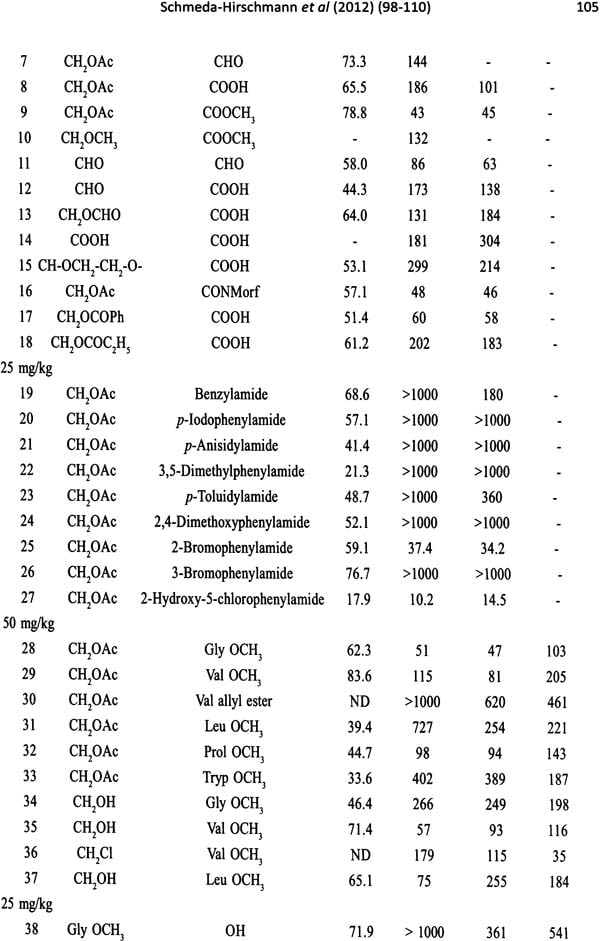
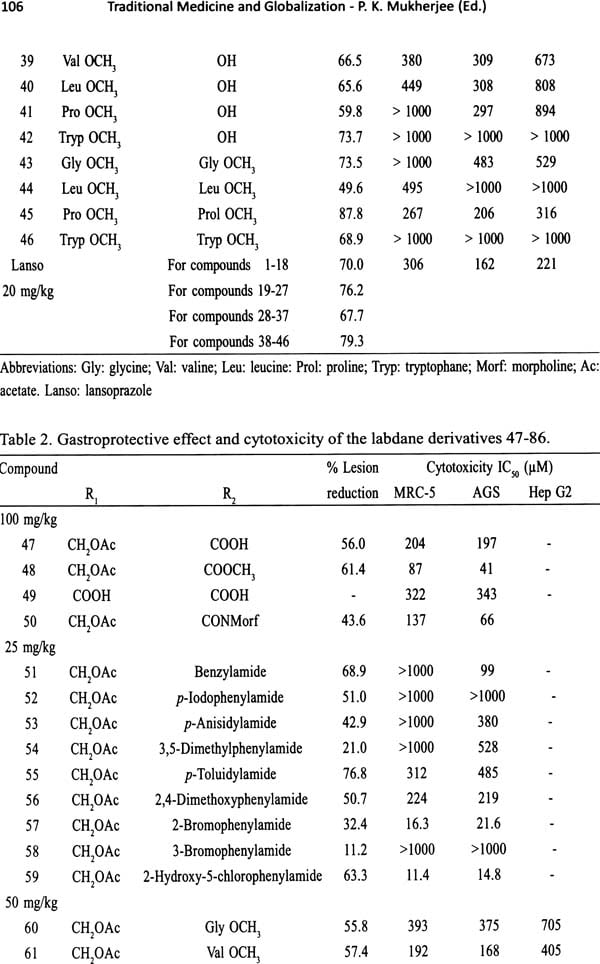
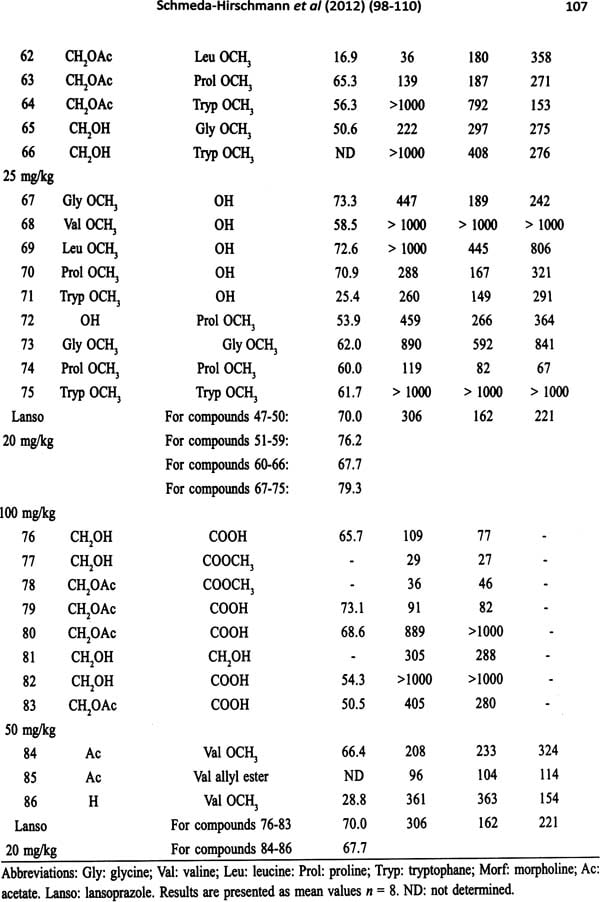
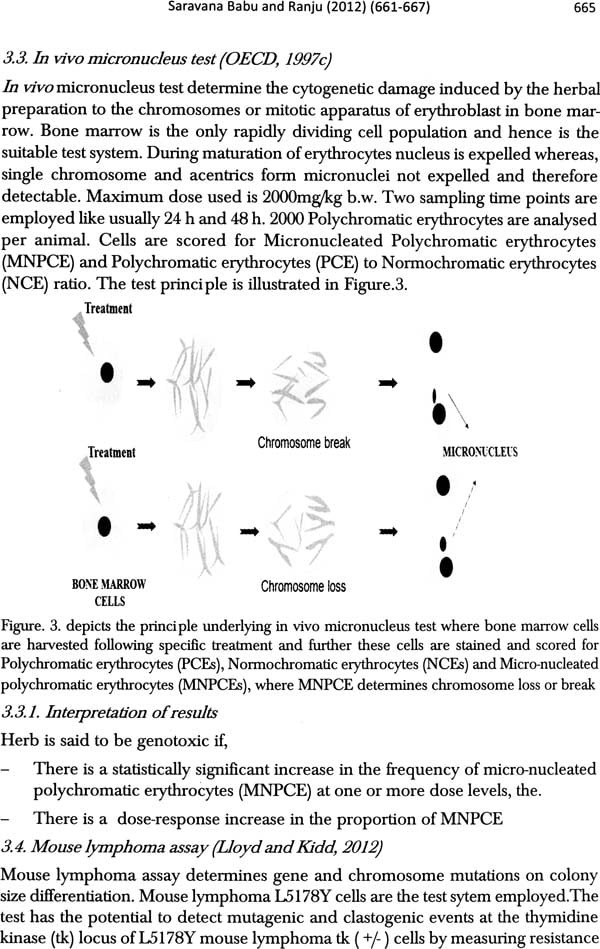
Traditional Medicine and Globalization- The Future of Ancient Systems of Medicine
Book Specification
| Item Code: | NAL646 |
| Author: | Pulok K. Mukherjee |
| Publisher: | Maven Publishers, Kolkata |
| Language: | English |
| Edition: | 2014 |
| ISBN: | 9788192624303 |
| Pages: | 740 |
| Cover: | Hardcover |
| Other Details | 9.5 inch x 7.0 inch |
| Weight | 1.60 kg |
Book Description
The development of natural products requires the confluence of the modem techniques and integrated approaches in various fields of science and technology. Intention of this book is to highlight different aspects of globalization of traditional medicine with the focus on some crucial and contemporary issues on validation of natural products. Eminent scientists around the globe have contributed for this book on different issues of etbnopharmacology, traditional medicine and globalization.
Given the importance on promotion and development of natural products, this book covers various aspects on revitalization of traditional medicines. The contributors have provided an insight into the modem aspects and future of traditional medicine inspired drug development, validation, safety related quality issues, sustainability and globalization. Bringing together expertise from academia and industry, this edited volume provides an overview of traditional medicine with wisdom and know-how in both innovation and commercialization, placing natural product research in a broader sense for readers.
This edited volume contains 68 chapters emphasizing several thrust areas of etbnopharmacology and natural products. A wide diversity of chapters has been contributed by eminent personalities throughout the world including Dr. A. P. J. Abdul Kalam, Sri. Shekhar Dutt, Dr. Marco Leonti, Dr. G. A. Cordell, Dr. R. Verpoorte, Dr. P. J. Houghton, Dr. P. Pushpangadan, Dr. Y. K. Gupta, Dr. A.K.S. Rawat, Dr. S.K. Kulkarni, Dr. Colin W. Wright, Dr. S. C. Mandal, Dr. C. K. Katiyar, Dr. T. K. Mukherjee, Dr. S. Rajan, Dr. G. Schemda, Dr. P. K. Debnath, Dr. Bob Alkin, Dr. Elin Y. Sukandar, Dr. N. Maity, Dr. A. K. Mitra, Dr. E. Hossain, Dr. H. Fukui, Dr. S.Bhadra, Dr. N. Neema, Dr. H. Mizuguchi, Dr. D.Chattopadhyay, Dr. T.Sen, Dr. Erdem Yesilada, Dr. S.K.Mitra, Dr. S.C. Bachar, Dr. S. Karmakar, Dr. G. B. J. Bailly, Dr. P. Haldar, Dr. Y. Oztruk, Dr. S. Pandit, and others. All representing the-state-of-the-art of the field, and thus would be interesting sources for information. This book will be of enormous help to the undergraduate and postgraduate students, researchers and academia and all others working in the area of Ethnopharmacology and natural product research. It will be an imperative addition to the libraries of technical and administrative personnel in the industries and Universities as a potential resource to update their knowledge.
Dr. Pubk K Mukherjee is working on traditional medicine inspired drug discovery leading to development of therapeutic leads from natural resources. His research work highlights on screening, evaluation, formulation and standardization of herbal drugs and their validation to ensure quality, safety and efficacy.
His research career has been outstanding, including globally acclaimed contributions on evaluation of the holistic medicine as useful bio-prospecting tools for the traditional medicine based drug discovery program so as to make them available from 'Farm to Pharma', Based on his works, he has to his credit numerous research publications in peer reviewed impact journals, several patents and books on evaluation of botanicals. He has developed several products, process with partnership between industry and institute.
For his excellent research career Dr Mukherjee has been awarded with so many laurels from Govt. of India and abroad; to name a few: he has been awarded with the prestigious Commonwealth Academic Staff Fellowship from Association of Commonwealth Universities [ACU], UK; Out Standing Service Award from Drug Information association [DIA], USA; Career Award for Young Teacher from All India Council for Technical Education (AICTE), Govt. of India; Overseas Award from Department of Biotechnology (DBT), BOYSCAST Fellowship from Department of Science & Technology (DST), Govt. of India; Young Pharmacy Teacher Award from Association of Pharmaceutical Teachers of India; IPA Fellowship Award from the Indian Pharmaceutical Association [IPA] and many others.
Dr. Mukherjee has been working for promotion of traditional Indian medicine to a great extent by dissemination of knowledge in the area of natural products particularly on promoting national/international collaboration and cooperation. Dr Mukherjee is serving as Associate Editor of the Journal of Ethnopharmacology; Elsevier Science. He is the member of the editorial board of several other Indian and international journals. He is also associated as advisor to different organizations and administrative bodies in India and abroad.
Few popular books authored/edited by Dr. Pulok K Mukherjee:
• "Evidence Based Validation of Herbal Medicines - Farm to Pharma"• Elsevier, USA (In Press)
• "Evaluation of Herbal Medicinal Products - Perspectives of Quality, Safety and Efficacy; Pharmaceutical Press, UK.
• "Quality Control on Herbal Drugs" - Business Horizons Ltd., India "Promotion and Development of Botanicals with International Coordination" - Allied Book Agency, India.
• "GMP in Herbal Drugs"- Business Horizons Ltd., India.
Traditional medicines (TM) are mostly indigenous to a country or region. It has developed through daily experiences and mutual relationships between people and nature. TM is gaining global acceptance as they offer natural ways to treat diseases and promote healthcare. Our common interest is to understand the cultural and therapeutic dimensions of medicinal plants used for healthcare around the globe. The development of TM requires the confluence of modern techniques and integrated approaches related to their research in various fields of science and technology. With all our involvement in drug development we should be realistic that the health problems of people worldwide cannot be solved exclusively by a single scientific academic field whatsoever, which require interdisciplinary research to make significant changes in the validation of natural products and its documentations at large. The promotion of indigenous medical system in primary healthcare is an important aspect, which should be explored with the development of international coordination and cooperation by the global use of traditional medicine.
This edited volume contains state-of-the-art reviews and research reports from eminent scientists throughout the world, based on their deliberations at the 12th International Congress of Ethnopharmacology, organized by the School of Natural Product Studies, Jadavpur University in Kolkata. The Congress evidenced the gracious presence of eminent scientists, academicians, industrialists, regulators, researchers and students from over 52 countries throughout the world including Dr. A. P. J. Abdul Kalam, former President of India, Prof. Luc Montagnier, Nobel Laureate in medicine and President, World Foundation for AIDS Research & Prevention - UNESCO, and many others.
This book mainly focuses on several aspects for revitalization of traditional medicine. This volume addresses several crucial and contemporary issues on globalization of traditional medicine and natural products by the renowned scientists throughout the world with major highlight on globalizing local knowledge and localizing global technologies. This edited volume contains 68 chapters emphasizing on several thrust areas as follows:
- Traditional medicine inspired drug discovery and development
- The interface between history and Ethnopharmacology of traditional medicines
- Validation of Ethnopharmacological claims
- Quality control and standardization of herbal medicine
- Polypharmacology of herbal medicine - use of medicinal plants in globalized era
- Phytochemical, pharmacological and clinical studies of natural products
- Promotion and development of Ayurveda and other system of medicine.
- Safety related quality issues on medicinal plants
- Ethnopharmacology, bio-cultural diversity and conservation of medicinal plants
- Harmonization of regulatory requirements to ensure quality, safety and efficacy
- Future of ancient system of medicines-multidisciplinary approaches in health sciences
Different chapters give an insight into contemporary scientific knowledge in the fields of natural product and Ethnopharmacology. The volume will be an indispensable resource offering multidisciplinary approaches on natural product for industrial and academic researchers, under-graduate and post-graduate students. It will be an imperative addition to the libraries of technical and administrative personnel in the industries and Universities as a potential resource to update their knowledge. It will also be an essential reference for anyone involved in the fields of phyto-medicine, traditional herbal remedies, pharmaceutical sciences and natural product research. I would like to extend my most cordial wishes to all the readers of this book.
Natural Products Research: Where will it go?
Robert Verpoorte
Natural Products laboratory, IBL, Leiden University, 2300 RA Leiden, The Netherlands &
Editor-in-Chief, Journal of Ethnopharmacology, Elsevier Sciences, The Netherlands
It is my great pleasure to introduce you to this book which bundles the papers presented at a meeting on medicinal plant research, organized in Kolkata, February 2012. As was clear at this meeting, we are in an exciting period for medicinal plant research. We are in a period in which the economic and cultural picture of the world is changing, a time in which many paradigms are changing, a time in which new approaches are being developed to better understand all kind of processes, being it biochemical, medical, biological or economical processes. Approaches which are more based on observations of systems, instead of a hypothesis, reductionist driven analysis of these processes.
In an historical perspective this is maybe not as surprising as it may have been at your first thought. Our ancestors did not have theories and hypotheses, they were very good observers with all their senses. Based on that, via trial and error, they developed all kind of things, from food and medicinal plants to dyes, fibers, construction materials, poisons for hunting and fishing, etc. They did not have GC, LC, MS or NMR, but were able to; for example, independently develop curare in the Amazon area, Central Africa and Malaysia, using different species from the same plant genus Strychnos. Also all caffeine containing plant species have been found by our ancestors all over the world and were used in all kinds of beverages, without any detector other than their own senses.
Natural products for health
The 40,000 to 70,000 plants that have a medicinal use somewhere in the world are also the result of our ancestors' efforts to cure diseases and improve their health (Verpoorte et al. 2006). The roots of all western medicines are in the Mediterranean and European region, with in the past centuries some imports from Asia and America. Although the statistics as reported by Newman and Cragg (2012) show that about half of all novel drugs that come to the market every year in the past 30 years are natural products or natural products derived, many of which are from microbial sources. Despite these hard facts, the screening of medicinal plants for novel drugs was not of high priority for the big pharmaceutical companies in this period. Reasons were the difficulties in obtaining material for screening in connection with the Rio de Janeiro treaty on biodiversity rights and possible future problems in sourcing of larger amounts of plants of interest. Moreover the elaborate approach of bioassay guided fractionation and loss of activity in this approach did not help in making medicinal plants a major natural resource in the lead finding strategies of the big pharma based on the paradigm of single compound - single target. However, times are changing, the patents on the major drugs are expiring, and the costs of developing novel drugs has gone up so much, that the amount of money available for R&D is not sufficient to cover for a new generation of blockbuster drugs. As a result, lead-finding facilities in the pharmaceutical industry are being cut; with the industry focusing on the tasks they are really good at the marketing. In fact almost half of the total budget of the pharmaceutical industry goes to selling/marketing and about 15% to novel drug development. In the future the big pharmaceutical companies will probably obtain most of their leads from small and medium size enterprises specialized in drug lead finding.
Traditional medicine
With the ongoing global changes, including the dissemination of knowledge via internet, the interest for other medical systems is growing worldwide, particularly the general public shows interest in natural medicines, believing that natural is a synonym for healthy. On the other hand the western medical practice and the medical sciences are not very open to consider these different systems as potential sources for getting new insights in the prevention and treatment of various diseases. The result of this dualism is an uncontrolled use of traditional/complementary medicines in the western world. Considering that most Western medicines have their roots in the Eurasian and Mediterranean traditions, respect and an open mind for the potential of other systems would be appropriate. That does not mean blind following, but unbiased studies of safety and efficacy of the traditional medicines, with as final goal evidence-based use and maybe new insights and leads for treating major diseases.
Novel approach
An important argument for such a novel approach is that the present drug development is stalling, it is based on the paradigm of "single compound – single target", that means a reductionist approach. That approach has been successful for a number of targets and diseases, in fact so successful that a much better drug for these targets are very difficult (and thus very costly) to find. The diseases still waiting for good cures or treatments are mostly multifactorial. It is thus unlikely that the mentioned paradigm will lead to a solution. This is where traditional medicine comes into the picture, as it represents a different, more holistic and personalized approach. The use of mixtures of plants, with clear roles ascribed to each of the ingredients, makes it plausible that this is a deliberate polypharmacological treatment. Such treatments may include the parallel interaction of different components in the mixtures with different targets, synergy, and/or suppression of side effects, in other words a multifactorial treatment for a multifactorial disease. The Asian medicinal concept of personalized medication is now also being studied and recognized as important (pharmacogenomics), though too expensive to implement in Western Medicine. The next step would be to also consider the multifactorial treatment as an interesting concept. That this can be successful can be learned from the treatment of HIV. This has improved enormously, among others, because of the application of mixtures of compounds. Pharmacologists have in fact already started now with what they call systems pharmacology.
In-vivo: systems biology
The major challenge we have now is to develop the tools to come to evidence based use of traditional medicines. That means the use of observation based systemic approaches, this could be in clinical trials for well known, commonly used medicinal plants (Anonymous, 2011; Verpoorte et al. 2005; Wang et al. 2005). Though in case of less well known plants proper bioassays are needed to first establish safety and activity, but in any case in-vivo systems will be needed to make a real systemic study of the effects. The omics will be the tools to observe the biological systems used to assay the activity. Metabolomics in combination with multivariate analysis are in this respect the key technologies. This systems biology approach is now gaining momentum (Verpoorte, 2005; Robinson and Zhang, 2011; Verpoorte 2012; Xu and Bauer, 2012 ), it also now referred to as reversed pharmacology of reversed drug discovery, starting form a traditional use working from clinical trial to lead, instead of from lead to clinical trial which was the approach of the past decades of drug development.
This book covers the field
In the book you will find a wide diversity of chapters, from more visionary to more focused on a specific plant. All representing the-state-of-the-art of the field, and thus would be interesting sources for inspiration for your research. I hope it will help in focusing all our research into evidence-based use of traditional medicine and from there to possible novel drugs. A focus which is needed to get all the data produced needed to get the traditional use approved by authorities like the FDA and EMA. Such registrations are urgently needed to overcome the skepticism of the western medical world and in that way make that governments are willing to give the necessary financial support for research projects in this field instead of just denying the existence of traditional medicines. At least form the meeting and the chapters in this book it is clear that in Asia traditional medicine is very much alive and being subject of many high level studies. Studies that eventually will pay off in new ideas and new medicines. I hope that this book may inspire the readers to further improve our research and thus contribute to improve our health and fight diseases. Finally I should like to thank the organizers of the meeting and the editors of this book for their great efforts to set these landmarks.